
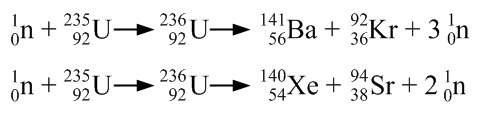
Show a water filled balloon – a good model for a nucleus.

In many ways, nuclei behave like a drop of liquid. any two smaller nuclei that can be made from the 235 nucleons of the 235 92U.Įpisode 527-2: Nuclear fission (Word, 123 KB) Demonstration: The nucleus as a liquid drop It absorbs a neutron, then splits into fission fragments, i.e. How do the two common isotopes of uranium 235 92U and 238 92U differ? ( 238 92U has three more neutrons than 235 92U.) It is the 235 92U not the 238 92U that fissions. The two lighter elements are referred to as fission fragments. It breaks up into two large chunks – into two elements nearer to the middle of the periodic table – so-called induced fission. The behaviour of the heaviest natural element, uranium, is different. In the examples above, small parts are chipped off nuclei. Students can practise balancing equations.Įpisode 527-1: Isotope production (Word, 50 KB) Discussion: Induced fission Ask your students to complete the following nuclear equation that summarises the transmutation of nitrogen into oxygen:Ĭockroft and Walton were the first to split the atom, by bombarding lithium with protons from their accelerator.ġ 1H + 7 3Li → 8 4Be → 2 4 2He Student questions: Balancing equations Patrick Blackett carried out further experiments and showed that the bombarded nucleus had transmuted. Rutherford found that protons exist in the nucleus by bombarding nuclei with alpha particles. There is another way in which an element may be transmuted for example, the production of radioactive 14 6C used in radio-carbon dating in the atmosphere by the neutrons in cosmic rays. Write general equations for these processes. (Moves one place down the periodic table.) What about β - decay? (Moves one place up the periodic table.) Introduce the idea of β + decay. Using a Periodic Table, explain that α decay moves two places down the periodic table. What is the nucleus made of? (Protons and neutrons, collectively know as nucleons.) What two natural processes change one element into another? ( α and β decay). Start by rehearsing some assumed knowledge. Student questions: Fission calculations (20 minutes).Discussion: The possibility of fission (10 minutes).Discussion and demonstrations: Controlled chain reactions (15 minutes).Worked example: A fission reaction (10 minutes).Discussion: Fission products and radioactive waste (10 minutes).Demonstration: The nucleus as a liquid drop (10 minutes).Discussion: Induced fission (10 minutes).Student questions: Balancing equations (30 minutes).Discussion: Transmutation of elements (15 minutes).There are other decay processes, and there are other events that occur when a nucleus absorbs a particle and becomes unstable. Students need to move beyond the idea that nuclear changes are represented solely by alpha, beta and gamma decay.


 0 kommentar(er)
0 kommentar(er)
